Cosmetics
A New Anti-Aging Technique Just Made It to Clinical Trials
Anti-Aging, Female Style
Menopause is a point of no return for women, considered irreversible until recently. Earlier in 2016, a team of experts was able to find a way to rejuvenate post-menopausal ovaries. After months of preclinical trials, experts from the Genesis Health Clinic in Athens are now launching the first clinical trials for the method.
The technique uses Platelet Rich Plasma (PRP) injections. But unlike other PRP transfusions, this one needs no donor. This PRP is made by centrifuging a person’s blood sample to isolate its growth factors. “It offers a window of hope that menopausal women will be able to get pregnant using their own genetic material,” Konstantinos Sfakianoudis, a gynecologist at Genesis, said.
The preclinical trials began in May 2016 and have yielded considerable results. PRP rejuvenated menopausal women’s ovaries, restoring fertility. Several were able to conceive after receiving PRP treatment. 75% of the 60 women treated became capable of conceiving through natural pregnancy or in vitro fertilization, and 9 actually got pregnant.
More than 75% showed overall hormone levels returned to youthful levels. It made women young again, so to speak.

Clinical Trials
Changing lifestyles have led to an increase in late pregnancies, and this comes with the usual complications associated with menopausal conception. Aside from this, there are also cases of women with difficulties in bearing children because of thin uterine lining. But after injecting PRP into the uterus of six women who had these conditions, they were able to bear children.
So this method doesn’t just restore a woman’s fertility, it can also alleviate some of the (many) negative effects associated with menopause. Konstantinos Pantos, Inovium Ovarian Rejuvenation Trials Director, explained:
“The goal of the trial is not to prove that we can reverse menopause, because over and over again in our treatments, we know that this is the result. We also know that the treatment triggers a whole body response that restores hormones to the levels of youth. Now, we want to see if the rejuvenation is a permanent one, and if we have discovered a connection between the loss of fertility and the damaging effects of aging in the body.”
The clinical trials have been approved by Greece’s National Ethics Committee and will start in February 2017. It isn’t free, however. Participation costs a hefty sum of $5,000. Similar trials are set to begin in multiple locations in the US by June 2017.
Source: 1
Researchers think they’ve finally figured out why bacteria only causes acne in some people

Despite the fact that up to 80 percent of us will experience the living nightmare that is acne at some point in our lives, scientists still don’t really understand what causes the condition, and more importantly, how to stop it.
But a new study might have finally figured out why skin bacteria only causes inflammation in some people and not others – and the discovery could lead to new acne treatments in as little as two years.
Right now, doctors treat severe acne with either antibiotics, hormone regulators (such as the contraceptive pill), or isoretinoin – better known as Roaccutane.
All of these come with side effects (some more severe than others), and worst of all, most of them don’t offer long-term relief, or in some cases, they don’t work at all.
It’s been years since any new types of acne treatments have been added – so new insight into the condition is long overdue.
The new study focussed on the fact that pretty much all of our skin is covered in bacteria all of the time – after all, it’s the first line of defence against invading germs.
But despite this constant coating of bacteria, many people never experience breakouts, while others can’t get rid of them no matter what they try.
“It’s a big puzzle as to why we tolerate all these bacteria on our skin,” lead researcher Richard Gallo from the University of California, San Diego, told MedicalXpress. “Usually, we walk around at peace with them. But at certain times, that detente breaks down and you get an infection.”
Now, for the first time, the team thinks they’ve discovered what causes this crucial difference.
Gallo and his colleagues showed that a usually harmless bacterium that lives on our skin starts triggering inflammation and breakouts when it finds itself trapped in airless, oily conditions, such as hair follicles.
But not everyone’s hair follicles are created equal, and that could explain why not everyone gets acne – some people might simply have hair follicles that are more suffocating than others.
This might not only explain the root causes of acne – it could also reveal a whole new pathway through which bacteria trigger inflammation, and that could help scientists understand a range of different infections.
The researchers specifically looked at a type of bacterium known as Propionibacterium acnes, which – as the name suggests – can cause acne breakouts.
Most of us have P. acnes on our face all the time, but it doesn’t always cause breakouts. So the team tested the bacteria under a range of conditions on the skin of mice to try and figure out what was going on.
They showed that when trapped in airless environments alongside hair and skin cells, P. acnes turned sebum – the oil found on our skin – into fatty acids that activate inflammation in nearby skin cells.
Usually this inflammation is switched off by enzymes called histone deacetylases, but the fatty acids produced by the bacteria deactivated that brake, so inflammation continued unchecked – going on to cause red, itchy breakouts.
So far, the research has only been done on mice, but the team is now looking to replicate their results in humans, and they’re hopeful that the inflammation pathway involved will be the same.
“For the first time, it shows how fatty acids derived from P. acnes act on skin cells to induce inflammation,” Holger Brüggemann, an expert on skin bacterium from Aarhus University in Denmark, who wasn’t involved in the study, told Andy Coghlan at New Scientist.
Brüggemann added that the new findings could also explain why teenagers are so prone to breakouts, because their sex hormones during puberty put their sebum production into overdrive, giving P. acnes more fuel.
The bad news is that cleaning your face regularly isn’t the answer, because the team showed that the bacteria clump together to form structures called biofilms, which effectively locks them onto your skin.
And, when this type of bacterium isn’t causing havoc inside suffocating hair follicles, P. acnes is actually beneficial to skin health, which explains why antibiotic treatments don’t work for many people – and in some cases, can actually make things worse.
But now that the team understands the root cause of the inflammation, they’re confident they’ll be able to come up with new treatments for acne.
“We can either inhibit these fatty acids, or block their impact on the skin,” Gallo told New Scientist. “We’re working on how to do this … If we get lucky, it could lead to new medications in two to five years.”
The researchers now want to investigate what it is specifically that makes some people’s faces more susceptible to acne. In addition to having particularly suffocating hair follicles, they might also be genetically disposed to being more vulnerable to the inflammation triggered by P. acnes fatty acids.
Or maybe the strains of bacteria they have on their skin make excessive amounts of fatty acids compared to other people’s strains. “I think all of these aspects probably play a role,” said Gallo.
Once they’ve figured this out, they’ll be a step closer to not only treating, but potentially preventing acne in the first place. Which would be a huge relief for all of us who’ve suffered the debilitating self-consciousness and pain of having breakouts.
The research has been published in Science Inflammation.
Source: 1
Scientists think they’ve found a hormone that reverses cell ageing in humans

Scientists have identified a male hormone that reverses cell ageing, potentially setting up new treatments to counter diseases caused by cells getting old and worn out.
The new clinical trial is the first time the use of hormones has been shown to reverse the ageing effects that happen naturally in human cells. We’ve not quite found a way to live forever, but the discovery could help some of us lead longer and healthier lives.
In the latest experiments, researchers from Brazil and the US used the steroid danazol, a synthetic male hormone, to stimulate the production of an enzyme called telomerase, already known to keep cells young by stopping the DNA inside them from shrinking.
They do this by stopping the degeneration of telomeres – the caps at the end of chromosomes (pictured in red above).
“One of the processes associated with ageing is progressive shortening of telomeres, DNA-protecting structures at the ends of chromosomes, like the plastic tips on shoelaces,” explained one of the researchers, Rodrigo Calado from the University of São Paulo in Brazil.
“Each time a cell divides, its telomeres get shorter,” Calado added. “Eventually, the cell can’t replicate anymore and dies or becomes senescent [biologically aged]. However, telomerase can keep the length of telomeres intact, even after cell division.”
Previous studies have shown how cell ageing can be stopped by increasing telomerase, which is naturally produced by cells that are constantly dividing, like blood-forming stem cells. A lack of it can affect internal organs and increase cancer risk.
This latest study proves that prescription steroids can generate telomerase on demand as well, confirming in the human body results that the team had previously seen in the lab.
Armed with this knowledge, new treatments for diseases like aplastic anaemia (where bone marrow stem cells age prematurely) could be developed, say the researchers. Pulmonary fibrosis, where the lungs become scarred, is another potential target.
In the study, a course of danazol was prescribed over two years for 27 patients suffering from aplastic anaemia, caused by telomerase gene mutations.
Typically over that time the average person would lose 100-120 telomere base pairs per year (the building blocks of DNA), but those with telomerase deficiencies might lose 200-600 base pairs over the same period.
When given the new treatment, the study participants’ cell telomere length not only stopped shrinking but increased by 386 base pairs on average.
On top of that, haemoglobin mass rose too, which meant patients were no longer dependent on blood transfusions.
Scientists have to progress cautiously though – the use of sex hormones comes with notable side effects, including mood swings, tiredness, and digestive system problems.
However, knowing how to combat one of the biological drivers of ageing is going to be valuable in all kinds of future research, even if an elixir of youth is out of reach for now.
The findings are published in the New England Journal of Medicine.
Source: 1
CR TidBit – BioEquivalence
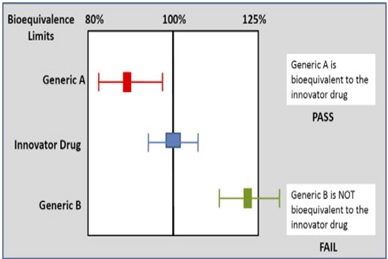
If two medicines are bioequivalent there is no clinically significant difference in their bioavailability. Although bioequivalence is most commonly discussed in relation to generic medicines, it is important to note that bioequivalence studies are also performed for innovator medicines in some situations such as:
- Between early and late clinical trial formulations or between the formulations used in clinical trials and the product to be marketed for new medicines.
- When changes in formulation have occurred after an innovator product has been approved, for example a change in one or more excipients (inactive ingredients).
Bioequivalence is determined based on the relative bioavailability of the innovator medicine versus the generic medicine. It is measured by comparing the ratio of the pharmacokinetic variables for the innovator versus the generic medicine.
The acceptance criteria are such that to be classified as bioequivalent, plasma concentrations of the generic medicine will not differ significantly compared with the innovator medicine. Studies have demonstrated that actual differences between observed mean plasma concentrations of generic and innovator medicines were no greater than 5%. In order to determine that two medicines are bioequivalent there must be no more than a 20% difference between the AUC and C max. This is based on international consensus that differences less than this are not clinically significant. In order to establish this, the AUC and C max for the generic medicine are compared to that for the innovator medicine.
Bioequivalence is based on a comparison of ratios where the ratio of generic to innovator for each pharmacokinetic variable does not differ by more than 8:10; this is how the range for the confidence intervals is defined:
8/10 = 0.80 gives the lower limit
10/8 = 1.25 gives the upper limit
The 90% confidence intervals for the ratios of both C max and AUC should be contained within the limits 0.80–1.25. Thus bioequivalence is based on ratios where the nominal equality is 1. It is not based on differences in absolute values. In practice, the generic product should have a ratio of mean values (AUC and C max generic: innovator) close to 1, indicating equality. If the observed ratio is closer to 0.8 or 1.25, then the data would have to contain little or no variation from the mean for the 90% confidence intervals of the ratio to lie in the 0.8 to 1.25 range that is necessary to demonstrate bioequivalence.
Reference: http://www.bpac.org.nz/BPJ/2009/generics/docs/bpjse_generics_bio_pages_4-8.pdf
CROs opt for computer model techniques to test cosmetics as ban on animal testing comes into effect
India’s pre-clinical contract research organisations (CROs) like Bioneeds, Advinus Therapeutics, Syngene and Jai Research Foundation are now looking at non-animal tests like the in-vitro analysis and even considering the advanced computer modelling techniques which are far more reliable to deliver human-relevant results in a day, unlike some animal tests that require a few weeks.
With the prohibition of the animal testing by the Union government and the revision of the Drugs and Cosmetics Rules 1945 inserting a new rule after 148-B with 148-C to forbid animal testing for cosmetics, research institutes like the IISc, NCBS and JNCASR point out that globally sophisticated computer models which accurately predict drug reactions, techniques like the 3-dimensional human cell derived skin model, quantitative structure activity relationships (QSARs) help to replace the use of guinea pigs or mice generating accurate allergic response data.
The departments of pharmacology in international universities have preferred the computerised human-patient simulators to indicate the adverse drug reactions. India with its scientific prowess and research capability could easily adopt the same, stated IISc, NCBS and JNCASR.
Bengaluru-based Bioneeds India, an OECD GLP Certified Pre-Clinical CRO, has already adhered to the European Commission’s Scientific Committee on Consumer Safety (SCCS) SCCS/1297/10 issued on 8 December, 2009 which mandates use of validated alternative methods in toxicological testing. These are Local Lymph node assay, NRU Photo toxicity, Bovine Corneal Opacity Study (BCOP), Dermal percutaneous absorption study (rHES), Direct Peptide Reactivity Assay (DPRA) and In vitro dermal irritation study (Epiderm).
“We anticipated this some time back following the EU directives and finally the Indian government has also taken a stand to implement and passing the message to the global regulators that; India is on par with the ‘Be Cruelty-Free Campaign’. Our scientific personnel are armed with the know-how for in-vitro tests and have pioneered in standardizing and implementing the same. In fact, we are one among the premier CROs in the country to comply with global regulatory guidelines of the European Commission’s SCCS which is still in the preliminary stage of implementation in India,” Dr. SN Vinaya Babu, managing director & chief executive officer, Bioneeds India Private Limited, told Pharmabiz.
However, it would have been better if this decision to ban animal studies for cosmetics becomes a global mandate. India, China and South East Asia are seen as hubs by the US and European Union for pre-clinical research. With the revival of the global economy, there would be a number of opportunities for companies in the region. Therefore adherence to SCCS norms are the need of the hour to grab some of the potential business opportunities, said Dr Babu.
Source: PharmaBiz