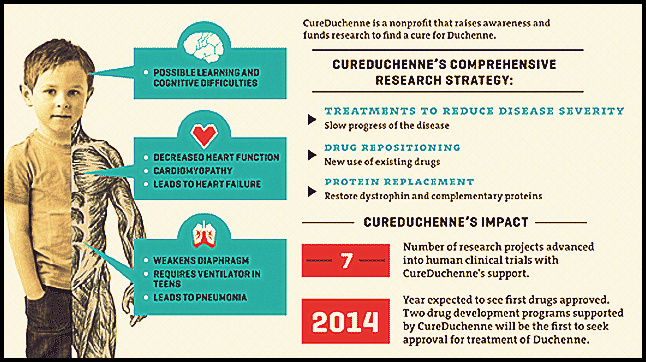
Duchenne muscular dystrophy
USFDA grants accelerated approval to first drug for Duchenne muscular dystrophy

The U.S. Food and Drug Administration approved Exondys 51 (eteplirsen) injection, the first drug approved to treat patients with Duchenne muscular dystrophy (DMD). Exondys 51 is specifically indicated for patients who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping, which affects about 13 percent of the population with DMD.
“Patients with a particular type of Duchenne muscular dystrophy will now have access to an approved treatment for this rare and devastating disease,” said Janet Woodcock, M.D., director of the FDA’s Center for Drug Evaluation and Research. “In rare diseases, new drug development is especially challenging due to the small numbers of people affected by each disease and the lack of medical understanding of many disorders. Accelerated approval makes this drug available to patients based on initial data, but we eagerly await learning more about the efficacy of this drug through a confirmatory clinical trial that the company must conduct after approval.”
DMD is a rare genetic disorder characterized by progressive muscle deterioration and weakness. It is the most common type of muscular dystrophy. DMD is caused by an absence of dystrophin, a protein that helps keep muscle cells intact. The first symptoms are usually seen between three and five years of age, and worsen over time. The disease often occurs in people without a known family history of the condition and primarily affects boys, but in rare cases it can affect girls. DMD occurs in about one out of every 3,600 male infants worldwide.
People with DMD progressively lose the ability to perform activities independently and often require use of a wheelchair by their early teens. As the disease progresses, life-threatening heart and respiratory conditions can occur. Patients typically succumb to the disease in their 20s or 30s; however, disease severity and life expectancy vary.
Exondys 51 was approved under the accelerated approval pathway, which provides for the approval of drugs that treat serious or life-threatening diseases and generally provide a meaningful advantage over existing treatments. Approval under this pathway can be based on adequate and well-controlled studies showing the drug has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit to patients (how a patient feels or functions or whether they survive). This pathway provides earlier patient access to promising new drugs while the company conducts clinical trials to verify the predicted clinical benefit.
The accelerated approval of Exondys 51 is based on the surrogate endpoint of dystrophin increase in skeletal muscle observed in some Exondys 51-treated patients. The FDA has concluded that the data submitted by the applicant demonstrated an increase in dystrophin production that is reasonably likely to predict clinical benefit in some patients with DMD who have a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. A clinical benefit of Exondys 51, including improved motor function, has not been established. In making this decision, the FDA considered the potential risks associated with the drug, the life-threatening and debilitating nature of the disease for these children and the lack of available therapy.
Under the accelerated approval provisions, the FDA is requiring Sarepta Therapeutics to conduct a clinical trial to confirm the drug’s clinical benefit. The required study is designed to assess whether Exondys 51 improves motor function of DMD patients with a confirmed mutation of the dystrophin gene amenable to exon 51 skipping. If the trial fails to verify clinical benefit, the FDA may initiate proceedings to withdraw approval of the drug.
The most common side effects reported by participants taking Exondys 51 in the clinical trials were balance disorder and vomiting.
The FDA granted Exondys 51 fast track designation, which is a designation to facilitate the development and expedite the review of drugs that are intended to treat serious conditions and that demonstrate the potential to address an unmet medical need. It was also granted priority review and orphan drug designation. Priority review status is granted to applications for drugs that, if approved, would be a significant improvement in safety or effectiveness in the treatment of a serious condition. Orphan drug designation provides incentives such as clinical trial tax credits, user fee waiver and eligibility for orphan drug exclusivity to assist and encourage the development of drugs for rare diseases.
The manufacturer received a rare pediatric disease priority review voucher, which comes from a program intended to encourage development of new drugs and biologics for the prevention and treatment of rare pediatric diseases. This is the seventh rare pediatric disease priority review voucher issued by the FDA since the program began.
Exondys 51 is made by Sarepta Therapeutics of Cambridge, Massachusetts.
EMA recommends conditional marketing authorisation for Translarna to treat Duchenne muscular dystrophy
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended granting a conditional marketing authorisation for Translarna (ataluren), an orphan-designated medicine for the treatment of Duchenne muscular dystrophy caused by nonsense mutations. Translarna is to be used in patients aged five years and older who are able to walk.
Duchenne muscular dystrophy is a genetic disease that gradually causes weakness and loss of muscle function. Patients with the condition lack normal dystrophin, a protein found in muscles. Because this protein helps to protect muscles from injury as muscles contract and relax, in patients with the disease the muscles become damaged and eventually stop working. There are currently no approved therapies available for this life-threatening condition and the current management of the disease is based on prevention and management of complications.
In the European Union (EU), approximately 18,600 people have Duchenne muscular dystrophy. The disease can be caused by a number of genetic abnormalities. Translarna is for use in the subgroup of patients whose disease is due to the presence of certain defects (called nonsense mutations) in the dystrophin gene, which prematurely stop the production of a normal dystrophin protein, leading to a shortened dystrophin protein that does not function properly. Translarna is thought to work in these patients by enabling the protein-making apparatus in cells to skip over the defect, allowing the cells to produce a functional dystrophin protein.
In January 2014, the CHMP originally adopted a negative opinion for Translarna, but at the request of the applicant, the CHMP started a re-examination of its opinion. Following careful consideration of all available evidence, including a re-analysis of the clinical data submitted by the company, the Committee concluded that the data available are sufficient to recommend a conditional marketing authorisation. Under the terms of the authorisation, the company will be required to provide comprehensive data from an ongoing confirmatory study.
Conditional marketing authorisation is an early access mechanism which allows the Agency to recommend marketing authorisation for medicines that address an unmet medical need for patients suffering from life-threatening diseases even if comprehensive clinical data are not yet available.
The applicant for Translarna is PTC Therapeutics Limited. The company is registered as a micro-, small- or medium-sized-enterprise (SME), and as such benefited from support and incentives offered by the Agency’s SME office.
Because Translarna has an orphan designation, the Agency provided free scientific advice to the applicant during the development of the medicine. Orphan designation and the associated incentives, such as free scientific advice or ‘protocol assistance’, are among the Agency’s most important instruments to encourage the development of medicines for patients suffering from rare diseases.
The CHMP opinion on Translarna will now be sent to the European Commission for adoption of a decision on an EU-wide marketing authorisation.
Source: PharmaBiz
Duchenne muscular dystrophy may be treated with erectile dysfunction drug
Approximately 1 in every 3,600 male infants worldwide is affected by Duchenne muscular dystrophy – an inherited condition that causes severe muscle weakness. At present, there is no specific treatment for the disease. But new research published in the journal Neurology suggests that certain drugs usually prescribed for erectile dysfunction may be effective.
Duchenne muscular dystrophy (DMD) is caused by a defective gene for dystrophin – a protein that helps maintain healthy muscles. Low levels or absence of dystrophin means the muscles lack nitric oxide – a chemical that signals blood vessels to dilate during exercise so blood flow can increase.
As a result of the way the gene is inherited, the condition primarily affects boys and young men. Onset of the disease usually occurs before the age of 6 years. As well as muscle degeneration, the condition can cause intellectual disability, congestive heart failure or irregular heart rhythm, back and chest deformities and respiratory disorders.
Many individuals with DMD are treated with corticosteroids. This medication can help to slow muscle degeneration and reduce negative effects on the heart and lung.
But the researchers of this study, including Dr. Ronald G. Victor of the Cedars-Sinai Heart Institute in Los Angeles, CA, note that corticosteroids cause an array of side effects and more than 75% of patients are unable to endure them.
With this in mind, the team set out to determine whether the drugs sildenafil (Viagra) or tadalafil (Cialis) could help treat DMD. Both drugs are commonly used to treat erectile dysfunction and pulmonary arterial hypertension. They work by relaxing blood vessels, therefore increasing blood flow.
Drugs improved blood flow in boys with DMD
For their study, the team assessed 10 boys aged between 8 and 13 years who had DMD. All boys were taking corticosteroids and were able to walk, although some often used a wheelchair or scooter to help with mobility.
The blood flow in the muscles of these participants was measured as they carried out a handgrip exercise and when they were resting.
Their blood flow was compared with 10 similarly aged healthy boys, which confirmed that those with DMD had abnormal blood flow, even when taking corticosteroids.
The boys with DMD were then required to take each drug – sildenafil or tadalafil – 2 weeks apart. Their blood flow was measured again as they rested and carried out a handgrip exercise, and this was compared with the blood flow of the 10 healthy boys.
The researchers found that after taking each drug, the boys with DMD had the same blood flow response as the healthy boys during exercise. The team notes that this result was instantaneous and was more pronounced when the drug was given in higher doses.
Dr. Victor notes that although a lot more research is to be done before either drug can be recommended for individuals with DMD, the team’s findings are encouraging. “This is not a cure, but it is the first stop toward identifying potential treatments,” he adds.
However, the researchers say their study has limitations. For example, they note that it is unknown whether long-term use of either drug can maintain improved blood flow.
Source: http://www.medicalnewstoday.com